Why Every Water Quality Analyst Needs a pH Meter FAQ Guide
Does your water pH analysis involve natural phenomena in aqueous solutions, chemical changes or manufacturing processes? Whether you're looking to purchase a pH meter for your lab or exploring new equipment ideas for your wastewater treatment plant, this FAQ guide has everything you need.
This guide provides detailed answers to the most frequently asked questions about pH meters. From understanding how pH meters work to understanding how to calibrate them, you'll find reliable answers tailored to professionals like you. pH meter for water is critically important and irreplaceable measuring instruments in modern science, industry, the environment, and everyday life. pH testers are centered on the importance of being able to quickly, accurately, and quantitatively measure the pH value of a solution.
We will also look at how choosing the right pH machine can improve the accuracy of your measurements while minimizing maintenance costs. If you are new to this field of water analysis, this guide will help you understand some basic considerations such as how to use a pH water meter, how to store electrodes, and how to buy a pH water tester.
Key topics covered include:
What are the categories of pH measuring instrument?
What are the appropriate electrodes for pH tester for water?
What factors affect the accuracy of pH meter measurements?
How to choose the best supplier for your lab?
Don't miss this opportunity to gain practical insights into getting the best digital pH meter for your needs. Read the pH Meter FAQ guide now to enhance your water quality analysis!
What Is pH Meter?
A pH meter is a scientific instrument used to measure the acidity or alkalinity of a solution by measuring the activity of hydrogen ions (H⁺) in the solution to determine its pH value. Water pH tester is like a sopHisticated “acidity detector”, which utilizes special glass electrodes to sense the “activity” of the hydrogen ions in the solution and converts this perception into a readily understood pH value on the display. The pH meter in water is like a sopHisticated “acidity detector” that uses special glass electrodes to sense the “activity” of hydrogen ions in a solution and translates this perception into a readily understandable pH value that is displayed, making it an indispensable and important tool in science and industry. For reliable results, proper calibration and electrode maintenance are essential.

What Is pH Value?
pH value, also known as acidity or alkalinity, is a ratio symbol used in chemistry to describe the degree of acidity or alkalinity of an aqueous solution.

What Does a pH Meter Measure?
The core function of a pH meter water is to measure the activity (concentration) of hydrogen ions (H⁺) in a solution and convert it to a pH value (range 0 to 14) to determine the strength of the acidity or alkalinity of the solution. It covers almost all liquid or soluble samples.

What Is a pH Meter Used For?
A pH test water is primarily used to accurately measure the pH of a solution. pH is a measure of the strength of the acidity or alkalinity of a solution, and usually ranges from 0 to 14:
pH < 7: Acidic solutions (High concentration of hydrogen ions)
pH = 7: neutral solution (e.g. pure water)
pH > 7: alkaline solution (low concentration of hydrogen ions, high concentration of hydroxide ions OH-)
How Does A pH Meter Work?
The pH water machine is based on the electrochemical principle that when the pH electrode is immersed in the solution to be measured, the difference in hydrogen ion concentration on both sides of the glass membrane of the measuring electrode produces a potential difference (potential).
This potential difference is proportional to the hydrogen ion concentration ratio (i.e. pH difference) between the solution to be measured and the buffer in the membrane and follows the Nernst equation.
The reference electrode provides a stable reference point.
The meter measures the total potential difference between the measuring electrode and the reference electrode.
The instrument's internal circuitry converts this millivolt voltage signal into a corresponding pH reading.

What Are The Main Components Of a pH Meter?
pH Electrode (Probe):
Glass Electrode: Its tip has a special sensitive glass membrane. When immersed in solution, the potential difference between the inside and outside of the membrane, generated by the difference in hydrogen ion concentration, changes.
Reference electrode: Provides a stable, known reference potential that is unaffected by the solution to be measured. Usually filled internally with an electrolyte (e.g. KCl) and in contact with the solution to be measured through a porous liquid junction.
Electronic meter: Amplifies the tiny voltage signal generated by the electrode and converts it into a pH reading that is displayed on a screen according to the Nernst equation. The instrument also has a temperature compensation function, data logging, and calibration point setting.

How To Calibrate pH Meter?
Prepare 1~3 kinds of standard buffer solution as needed (according to the number of points of calibration);
Clean the pH electrode and temperature electrode with distilled water, and put them into the pH standard buffer solution;
If the instrument has buffer solution automatic recognition function, it will automatically recognize the standard solution and display the nominal value; if the recognition is not successful, it may be due to electrode damage, temperature sensor damage, etc., and the user should check it carefully. If it has been unsuccessful, it can also be calibrated by manual recognition.
In case of manual recognition, it is necessary to input the nominal value manually, i.e. every time when calibrating the standard buffer solution, manually input the pH nominal value of the current standard solution corresponding to the current temperature.
If the standard solution cannot be recognized, or the nominal value is not entered, this calibration will not continue.
After the above steps are completed and the reading is stabilized, press the “Calibration” or “Confirmation” button (the names of the buttons are different for different instruments), the instrument will store the calibration results and display the calibration results;
If you need to continue calibrating other standard solutions, clean the electrode and put it into another standard buffer solution. If you need to continue to calibrate other standard solution, you need to clean the electrode and put it into another standard buffer, also wait until the instrument recognizes successfully and the reading stabilizes, then press “Calibrate” key or “Confirm” key to finish the calibration.
After the final calibration, the instrument interface usually displays the calibration result, i.e. pH slope, which indicates the amount of potential change per 1 pH change, usually expressed in mV/pH or %.

How To Use pH Meter?
Power on
Check whether the electrode is intact, new electrode or electrode not used for a long time must be soaked in distilled water for several hours before use.
Press the power switch and warm up for 5 minutes.
Calibration
Calibration is a critical step to ensure the accuracy of the measurement and generally requires at least two standard buffer solutions, e.g., pH 7.00 and 4.01 buffer solutions. After completing the first point of calibration, clean the electrode and place it in another pH buffer solution for the second point of calibration.
Determination of solution pH
The electrode was first cleaned with distilled water and then once more with the solution under test.
Stir the solution with a glass rod to make the solution homogeneous, immerse the electrode in the measured solution and read out its pH value. When measuring, the electrode bulb should be fully immersed in the measured solution, with the electrode 1-2cm away from the container, avoiding touching the bottom and side walls of the beaker.
Wait for the reading to stabilize before reading the data.
End operation
Wash the electrode with distilled water and absorb it with filter paper.
Put on the composite electrode cover, a small amount of replenishment liquid should be put inside the cover.
Unplug the composite electrode and connect a short wire to prevent dust from entering and affecting the accuracy of measurement.

What Is The Application Of pH Meter?
Laboratory research: solution pH monitoring in chemistry, biology, environmental science and other experiments.
Water quality monitoring: pH testing of drinking water, wastewater, rivers, lakes, swimming pool water.
Industrial production process control: chemical, pHarmaceutical, food and beverage (such as beer, fruit juice), electroplating and other industries in the reaction process of pH regulation and monitoring.
Agriculture and soil science: Measurement of soil pH, guidance for fertilization.
Food processing and safety: Control of acidity in food products (e.g. cheese, yogurt, canned goods).
Aquaculture: Maintaining optimal pH for aquatic organisms such as fish.
Education: Laboratory teaching in school chemistry and biology programs.

Why Is The Temperature Given On The pH Meter?
If the pH test water is calibrated at a different temperature than the temperature at which the sample was measured, and the instrument is not temperature compensated, then the instrument's built-in calibration curve will no longer be applicable to the sample measurement, resulting in a significant measurement error. The greater the temperature change, the greater the potential error.
Temperature dependence of the electrodes themselves:
In addition to theoretical slope changes, the internal systems of the glass film electrodes and reference electrodes also change slightly with temperature.
This inherent temperature dependence is usually expressed as the “isothermal point” or “zero potential point” of the electrode. Most pH electrodes are relatively insensitive to temperature changes near pH=7 (minimal change in slope), but away from 7, the error due to temperature changes increases.
Temperature changes also affect the response time of the electrode. Typically, an increase in temperature will speed up the response and a decrease in temperature will slow it down.
The pH of buffers and samples varies with temperature:
The pH of the buffer solution used to calibrate the digital pH tester is itself a function of temperature. Example:
Effects: Calibration must be performed at the current temperature using the pH of the standard buffer corresponding to that temperature or the calibration base point is incorrect.
The sample itself: The chemical equilibrium of the solution shifts with temperature, causing the true pH of the solution to change.

Can a pH Meter Measure Conductivity?
The lab pH meter itself cannot directly detect conductivity
Incompatible electrode structures
The glass membrane of pH electrodes responds selectively to hydrogen ions, whereas conductivity electrodes require an inert sheet of metal (e.g., platinum) in direct contact with the solution to form a current loop.
Measurement Circuit Differences
pH-meters detect weak voltage signals with high impedance (millivolts), conductivity meters measure current/resistance with low impedance.
Functional Design Limitations
The standard pH instrument mainframe does not have a conductivity measurement module or algorithm.

Can a Soil pH Meter Be Used For Water?
It can, but there are limitations and risks to accuracy. A soil pH meter is essentially a specialized pH electrode designed with the soil environment in mind.
Soil pH meters can provide a rough measurement of water pH, but are limited to emergencies or scenarios where accuracy is not critical.
After measuring acidic soils (pH<5.5), the electrode needs to be additionally immersed in a weak alkaline solution (pH 9~10) to prevent the “acid memory effect”;
Long-term use in water measurements accelerates electrode deterioration, so it is recommended that a dedicated device be used for this purpose!

How Much Does a pH Meter Cost?
It wouldn’t be right to mention one fixed amount.
The pH meter price will vary from model to model.
To know the exact price, you can ask your preferred manufacturer to provide a quotation for the type of good pH meter you want.
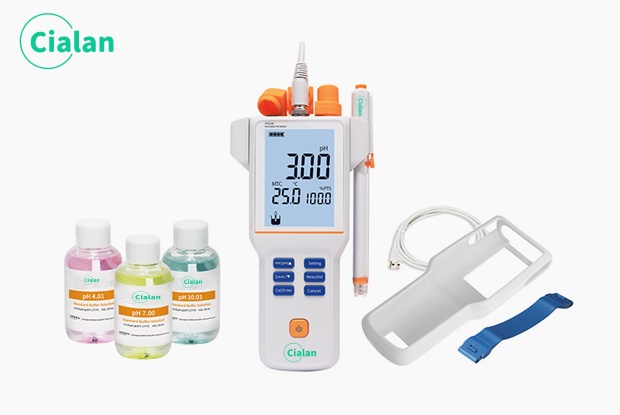
How Often Should You Calibrate a pH Meter?
The calibration frequency is not a fixed value, the recommendations are:
Laboratory: Before each experiment
Industrial: At the beginning of each shift + midway through slope verification
Field: First time of day + in case of abnormal readings
Accurate recording of slope and temperature for each calibration is more important than simply increasing the frequency!

How To Clean The pH Meter/Probe/Electrodes?
After contamination of the bulb and the liquid junction, the electrode is first cleaned with the following solvents, then the solvent is washed off with deionized water, and the electrode is immersed in the soaking solution for activation.
pH pollutant cleaning agent:
Inorganic metal oxides less than 1 mol / L dilute acid; organic oil and grease substances dilute detergent (weak acidic); resin polymer substances dilute alcohol, acetone, ether; protein hemocyte precipitates acid enzyme solution (such as the mother of raw tablets) pH pigmented substances dilute bleach, hydrogen peroxide.

Which Electrode Used In pH Meter?

How Long Does A Typical pH Meter Electrode Last?
The normal use of pH meter electrode/probe is half a year to one year, the use of not good condition of industrial electrode is shorter.
The life of the electrode is affected by the following factors
Using medium: the same electrode will contact with different mediums, some mediums are very clean, such as tap water, and some mediums are strong acid and alkali or even high temperature.
Time of use: occasional use and frequent use will lead to different life cycle of the electrode.
Whether to clean and maintain: can significantly extend its life and ensure data reliability.

How Should I Store My Electrode?
1. When the instrument is not in use, rinse the electrode with distilled water to minimize contamination and keep the pH probe in a protective cover with HI70300 storage solution. Do not soak or store the electrode in distilled or deionized water.
2. If the pH electrode is dry, soak it in HI70300 storage solution for at least one hour to reactivate the electrode.
3. To prolong the life of the pH electrode, it is recommended that it be immersed in an electrode cleaning solution for a half-hour soak and clean every month, then rinsed clean again with distilled water and recalibrated for storage.

How To Maintain pH Meter?
Daily maintenance of aquarium pH meter includes:
Make sure the sponge inside the cap of the electrode moist, add a few drops of pure water if dry.
When the electrode head is dry, it can be immersed in maintenance solution for a few hours;
Wash the electrode head after use and keep it clean;
Avoid hitting the electrode head;
Calibrate the electrode head regularly, it is recommended to calibrate the electrode head once a week, and daily calibration is required for everyday use;
Soak the electrode head in the maintenance solution regularly to keep it in an activated state.

How To Store And Use pH Standard Buffer Solution?
Preservation: glass bottle/polyethylene bottle (PE bottle for alkaline buffer solution), sealed and refrigerated (5~10℃), shelf life of about 6 months.
-Discard when turbid, precipitated or moldy.
-Use: Dispense into 50mL vials, equilibrate at room temperature for 1~2 hours and then use to avoid contamination by pouring back into larger vials.
- pH 9.18, 10.01 is easy to absorb CO2, it is recommended to use up within 2~3 days; pH 7.00 and other higher stability.

How To Make Buffer Solution For pH Meter?
When preparing buffer solutions, the deionized water used to make the solution should be boiled for 15-30 minutes to remove dissolved carbon dioxide. Avoid contact with air during cooling to prevent contamination by carbon dioxide.
The buffer solution should be stored in glass or polyethylene bottles. The lid should be tightly closed and the bottle stored in a low-temperature environment of 5-10°C. It can be stored for about six months. If turbidity, mildew, or sedimentation is found, it should not be used. Buffer solutions prepared in an environment higher than 10°C can be used for two to three days. Solutions with a pH of 7.00, 6.86, or 4.00 can be used a little longer. Due to the absorption of carbon dioxide in the air, solutions with a pH of 9.18 or 10.01 are more likely to change pH values, so it is recommended to use them as soon as possible.
When using the buffer solution, pour the solution into a 50 mL polyethylene vial and leave it at ambient temperature for 1-2 hours to equilibrate before use. Do not re-pour the solution from the vial into a larger vial to avoid contamination of the reagents.

What Do You Use To Calibrate a pH Meter
Calibration of electronic pH meter must be done using standard buffer solutions (Buffer Solutions) that are highly pH stable and certified to international standards at specific temperatures. pH 7.00 + (4.01 or 10.01) → covers 90% of application scenarios.

What Is ATC In pH Meter?
ATC: Automatic temperature compensation: Laboratory pH meters are equipped with automatic temperature compensation. This is usually accomplished by a separate temperature probe (or integrated in a composite electrode). The probe measures the actual temperature of the solution, T. The instrument calculates the theoretical slope at that temperature (2.303 RT / F) in real time based on the Nernst equation and adjusts its internal calculations accordingly.
The ATC compensates for the change in the slope of the electrode response with temperature. It ensures that the potential-pH relationship curve established by the instrument during calibration scales correctly to the current temperature.
What Is mV In pH Meter?
mV is the voltage signal directly generated by the pH electrode that reflects the H⁺ activity of the solution. The mV (millivolt) that appears in pH measurement refers to the voltage signal output from the pH electrode, which is the core raw data of pH measurement. pH value, which is the value of the instrument internally based on the millivolt (mV) voltage signal generated by the electrode, after complex temperature compensation, Nernst's equations, and calibration data, will be converted from the measured mV value to the pH value required by the user.
What Is ORP In pH Meter?
ORP, or Oxidation Reduction Potential, is a parameter independent of pH, but is strongly influenced by pH.
It is measured using an ORP electrode (platinum electrode) and the result, expressed in mV, reflects the strength of the oxidizing or reducing properties of the solution.
On a multi-function water analyzer or digital pH test meter with mV mode, the mV reading displayed when the ORP electrode is connected or switched to the ORP/mV function is the ORP value.

What Is The Difference Between a pH Meter And a pH Paper?

What Are The Main Types Of pH Meter?

What Factors Affect pH Measurement Accuracy?
- Electrode Aging: After a period of time, the glass membrane of a pH electrode will gradually age, resulting in a slower response time of the electrode and a decrease in the accuracy and stability of the measurement. For example, an electrode used for many years may take longer time to reach a stable potential value, making the measurement results biased.
- Electrode contamination: If the electrode surface is contaminated by oil, protein, particles, etc., it will affect the exchange process of hydrogen ions, thus affecting the measurement results.
- Inaccurate instrument calibration: pH machine water needs to be calibrated regularly with standard buffer solution, if the calibration is inaccurate, the measurement results will produce errors
- Temperature: Temperature has a significant effect on pH measurement. On the one hand, temperature affects the slope of the electrode, and on the other hand, temperature changes the equilibrium constant of the chemical reaction in solution, thus affecting the hydrogen ion concentration
- Ionic Strength: The ionic strength of a solution affects the activity coefficient and therefore the pH measurement. When the ionic strength of the sample solution differs significantly from the ionic strength of the calibration solution, the measurement results will be in error. For example, when measuring the pH of a high-salt solution, the measured value may deviate from the actual value due to the ionic strength.
- Homogeneity of the sample: If the sample is not homogeneous, such as the presence of precipitation, stratification and other phenomena, it will lead to inaccurate measurement results. When measuring, it is necessary to ensure that the sample is well mixed. For example, when measuring soil leachate containing suspended particles, if it is not shaken well, the measurement results may vary depending on the sampling location.
- Measurement time: Measurement needs to wait for the electrode to reach a stable potential value, if the measurement time is too short and the electrode has not reached equilibrium, the measurement result will be inaccurate. If the measurement time is too short, the electrode has not reached equilibrium, the measurement results will be inaccurate. Different solutions and electrodes reach equilibrium in different times, generally need to wait for several minutes
- Stirring speed: It is necessary to control the appropriate stirring speed during the measurement.
- Depth of electrode insertion: The depth of electrode insertion into the solution will also affect the measurement results. The electrode should be inserted into the solution at a depth where the glass membrane is completely submerged.

What Is The Precautions When Using pH Meter?
1. The electrode socket should be kept dry.
2. The input should be short-circuited when measurement is not carried out.
3. New electrodes or electrodes that have not been used for a long time must be soaked in distilling water for a few hours before using.
4. The electrode bulb should be fully immersed in the solution to be measured when measurement is carried out.
5. When used, the internal reference electrode should be immersed in the internal reference solution.
6.When using, the rubber plug of the electrolyte filling port of the reference electrode should be pulled out.
7. Potassium chloride salt bridge solution should be added frequently to keep the liquid level higher than the silver/silver chloride wire.

How To Buy An pH Meter From China?
Importing high performance electronic pH tester from China can be difficult for those who are new to international imports.
Purchasing from a reputable manufacturer and supplier eliminates hassle of logistics, as they handle the safe and reliable delivery of the product directly to your place.
When buying the pH meter instrument, it is important to keep the following points in mind:
1. Confirm that your selected supplier can securely export and deliver the machine, and ensure that your agreement includes all essential shipping information.
2. Choose the delivery way (by sea, air, or express) based on your preference.
3. Remember to factor in duty imports and taxes when calculating the total cost.

How Does a Manufacturer Deliver The pH Meter?
Safe shipping is so important as quality manufacturing.
A responsible manufacturer should deliver your rapitest pH meter safely.
Most water pH meter manufacturers use export cartoon case to pack the device.
You should read all the necessary instructions available on the sides of the boxes before boxing.

Is There Any Warranty On pH Meter?
For optimal performance, it is important to regularly maintain your food pH meter, so it can operate efficiently for an extended period. It is essential to be aware of the warranty coverage for your equipment, as it ensures that any manufacturing issues will be repaired at no additional cost by the supplier. Therefore, it is recommended to purchase a pH meter near me from a manufacturer that offers warranty services —Cialan.
Reputable digital water pH tester manufacturers typically provide a 1-year warranty. for example, offers complimentary repair and maintenance services during the first year.
Before purchasing a pH water meter, it is advisable to review the warranty period and other associated terms and conditions.
What After-Sale Service Does A pH Meter?
The reliable China digital pH test meter suppliers offers the following after-sale services:
1. Secure product delivery.
2. Thorough inspection prior to installation.
3. Complimentary online guidance for device installation and maintenance.
4. Free training for your users.
5. Regular assessments of your electronic pH meter.
Can You Do OEM&ODM For pH Meter?
Yes, Cialan can do OEM#OEM for our users, just send us your requirements for pool ph meter.

Where To Find pH Meter Manufacturers?
Numerous online directories can connect you with electronic pH tester manufacturers.
A simple search for "top-rated pH test meter manufacturers" yields multiple options. However, it's essential to exercise caution when selecting a manufacturer from online search results. Before making a decision, conduct thorough research on the company's background and reputation. Look up reviews on “Google” and reputable review sites to gauge their credibility.
Additionally, It's also crucial to review their portfolio of request that potential manufacturers share examples of their previous work to ensure they meet your standards.
How To Choose A Right pH Meter?
1. Testing accuracy
This is an important parameter of a water pH machine. Small number, high accuracy.
2. Testing range
The pH measurement range varies greatly for different samples and application scenarios. When purchasing, please make sure that the measurement range of the pH meter can cover the possible pH range of the sample to be measured, so as to avoid the situation of not being able to measure accurately due to out-of-range.
3. Electrode performance
Common electrodes include glass electrodes and composite electrodes. Glass electrodes react strongly to pH but are fragile; composite electrodes are the most common, integrating the measurement electrode and reference electrode together, easy to use and suitable for most aqueous solutions.
4. Calibration function
The instrument can automatically recognize the standard buffer and carry out calibration, which is very convenient and reduces human error. It can also be calibrated at single and multiple points to meet different measurement needs.
5. Ease of use
Simple operation interface, easy to read, portable and easy to maintain.

What Should You Look For In The Best pH Meter?
The best pH meter is one that is stable, reliable, easy to operate, rugged (for your environment), at the level of accuracy you need, and has reasonable maintenance costs.
This guide provides detailed answers to the most frequently asked questions about pH meters. From understanding how pH meters work to understanding how to calibrate them, you'll find reliable answers tailored to professionals like you. pH meter for water is critically important and irreplaceable measuring instruments in modern science, industry, the environment, and everyday life. pH testers are centered on the importance of being able to quickly, accurately, and quantitatively measure the pH value of a solution.
We will also look at how choosing the right pH machine can improve the accuracy of your measurements while minimizing maintenance costs. If you are new to this field of water analysis, this guide will help you understand some basic considerations such as how to use a pH water meter, how to store electrodes, and how to buy a pH water tester.
Key topics covered include:
What are the categories of pH measuring instrument?
What are the appropriate electrodes for pH tester for water?
What factors affect the accuracy of pH meter measurements?
How to choose the best supplier for your lab?
Don't miss this opportunity to gain practical insights into getting the best digital pH meter for your needs. Read the pH Meter FAQ guide now to enhance your water quality analysis!

What Is pH Meter?
A pH meter is a scientific instrument used to measure the acidity or alkalinity of a solution by measuring the activity of hydrogen ions (H⁺) in the solution to determine its pH value. Water pH tester is like a sopHisticated “acidity detector”, which utilizes special glass electrodes to sense the “activity” of the hydrogen ions in the solution and converts this perception into a readily understood pH value on the display. The pH meter in water is like a sopHisticated “acidity detector” that uses special glass electrodes to sense the “activity” of hydrogen ions in a solution and translates this perception into a readily understandable pH value that is displayed, making it an indispensable and important tool in science and industry. For reliable results, proper calibration and electrode maintenance are essential.

What Is pH Value?
pH value, also known as acidity or alkalinity, is a ratio symbol used in chemistry to describe the degree of acidity or alkalinity of an aqueous solution.

What Does a pH Meter Measure?
The core function of a pH meter water is to measure the activity (concentration) of hydrogen ions (H⁺) in a solution and convert it to a pH value (range 0 to 14) to determine the strength of the acidity or alkalinity of the solution. It covers almost all liquid or soluble samples.

What Is a pH Meter Used For?
A pH test water is primarily used to accurately measure the pH of a solution. pH is a measure of the strength of the acidity or alkalinity of a solution, and usually ranges from 0 to 14:
pH < 7: Acidic solutions (High concentration of hydrogen ions)
pH = 7: neutral solution (e.g. pure water)
pH > 7: alkaline solution (low concentration of hydrogen ions, high concentration of hydroxide ions OH-)

How Does A pH Meter Work?
The pH water machine is based on the electrochemical principle that when the pH electrode is immersed in the solution to be measured, the difference in hydrogen ion concentration on both sides of the glass membrane of the measuring electrode produces a potential difference (potential).
This potential difference is proportional to the hydrogen ion concentration ratio (i.e. pH difference) between the solution to be measured and the buffer in the membrane and follows the Nernst equation.
The reference electrode provides a stable reference point.
The meter measures the total potential difference between the measuring electrode and the reference electrode.
The instrument's internal circuitry converts this millivolt voltage signal into a corresponding pH reading.

What Are The Main Components Of a pH Meter?
pH Electrode (Probe):
Glass Electrode: Its tip has a special sensitive glass membrane. When immersed in solution, the potential difference between the inside and outside of the membrane, generated by the difference in hydrogen ion concentration, changes.
Reference electrode: Provides a stable, known reference potential that is unaffected by the solution to be measured. Usually filled internally with an electrolyte (e.g. KCl) and in contact with the solution to be measured through a porous liquid junction.
Electronic meter: Amplifies the tiny voltage signal generated by the electrode and converts it into a pH reading that is displayed on a screen according to the Nernst equation. The instrument also has a temperature compensation function, data logging, and calibration point setting.

How To Calibrate pH Meter?
Prepare 1~3 kinds of standard buffer solution as needed (according to the number of points of calibration);
Clean the pH electrode and temperature electrode with distilled water, and put them into the pH standard buffer solution;
If the instrument has buffer solution automatic recognition function, it will automatically recognize the standard solution and display the nominal value; if the recognition is not successful, it may be due to electrode damage, temperature sensor damage, etc., and the user should check it carefully. If it has been unsuccessful, it can also be calibrated by manual recognition.
In case of manual recognition, it is necessary to input the nominal value manually, i.e. every time when calibrating the standard buffer solution, manually input the pH nominal value of the current standard solution corresponding to the current temperature.
If the standard solution cannot be recognized, or the nominal value is not entered, this calibration will not continue.
After the above steps are completed and the reading is stabilized, press the “Calibration” or “Confirmation” button (the names of the buttons are different for different instruments), the instrument will store the calibration results and display the calibration results;
If you need to continue calibrating other standard solutions, clean the electrode and put it into another standard buffer solution. If you need to continue to calibrate other standard solution, you need to clean the electrode and put it into another standard buffer, also wait until the instrument recognizes successfully and the reading stabilizes, then press “Calibrate” key or “Confirm” key to finish the calibration.
After the final calibration, the instrument interface usually displays the calibration result, i.e. pH slope, which indicates the amount of potential change per 1 pH change, usually expressed in mV/pH or %.

How To Use pH Meter?
Power on
Check whether the electrode is intact, new electrode or electrode not used for a long time must be soaked in distilled water for several hours before use.
Press the power switch and warm up for 5 minutes.
Calibration
Calibration is a critical step to ensure the accuracy of the measurement and generally requires at least two standard buffer solutions, e.g., pH 7.00 and 4.01 buffer solutions. After completing the first point of calibration, clean the electrode and place it in another pH buffer solution for the second point of calibration.
Determination of solution pH
The electrode was first cleaned with distilled water and then once more with the solution under test.
Stir the solution with a glass rod to make the solution homogeneous, immerse the electrode in the measured solution and read out its pH value. When measuring, the electrode bulb should be fully immersed in the measured solution, with the electrode 1-2cm away from the container, avoiding touching the bottom and side walls of the beaker.
Wait for the reading to stabilize before reading the data.
End operation
Wash the electrode with distilled water and absorb it with filter paper.
Put on the composite electrode cover, a small amount of replenishment liquid should be put inside the cover.
Unplug the composite electrode and connect a short wire to prevent dust from entering and affecting the accuracy of measurement.

What Is The Application Of pH Meter?
Laboratory research: solution pH monitoring in chemistry, biology, environmental science and other experiments.
Water quality monitoring: pH testing of drinking water, wastewater, rivers, lakes, swimming pool water.
Industrial production process control: chemical, pHarmaceutical, food and beverage (such as beer, fruit juice), electroplating and other industries in the reaction process of pH regulation and monitoring.
Agriculture and soil science: Measurement of soil pH, guidance for fertilization.
Food processing and safety: Control of acidity in food products (e.g. cheese, yogurt, canned goods).
Aquaculture: Maintaining optimal pH for aquatic organisms such as fish.
Education: Laboratory teaching in school chemistry and biology programs.

Why Is The Temperature Given On The pH Meter?
If the pH test water is calibrated at a different temperature than the temperature at which the sample was measured, and the instrument is not temperature compensated, then the instrument's built-in calibration curve will no longer be applicable to the sample measurement, resulting in a significant measurement error. The greater the temperature change, the greater the potential error.
Temperature dependence of the electrodes themselves:
In addition to theoretical slope changes, the internal systems of the glass film electrodes and reference electrodes also change slightly with temperature.
This inherent temperature dependence is usually expressed as the “isothermal point” or “zero potential point” of the electrode. Most pH electrodes are relatively insensitive to temperature changes near pH=7 (minimal change in slope), but away from 7, the error due to temperature changes increases.
Temperature changes also affect the response time of the electrode. Typically, an increase in temperature will speed up the response and a decrease in temperature will slow it down.
The pH of buffers and samples varies with temperature:
The pH of the buffer solution used to calibrate the digital pH tester is itself a function of temperature. Example:
Effects: Calibration must be performed at the current temperature using the pH of the standard buffer corresponding to that temperature or the calibration base point is incorrect.
The sample itself: The chemical equilibrium of the solution shifts with temperature, causing the true pH of the solution to change.

Can a pH Meter Measure Conductivity?
The lab pH meter itself cannot directly detect conductivity
Incompatible electrode structures
The glass membrane of pH electrodes responds selectively to hydrogen ions, whereas conductivity electrodes require an inert sheet of metal (e.g., platinum) in direct contact with the solution to form a current loop.
Measurement Circuit Differences
pH-meters detect weak voltage signals with high impedance (millivolts), conductivity meters measure current/resistance with low impedance.
Functional Design Limitations
The standard pH instrument mainframe does not have a conductivity measurement module or algorithm.

Can a Soil pH Meter Be Used For Water?
It can, but there are limitations and risks to accuracy. A soil pH meter is essentially a specialized pH electrode designed with the soil environment in mind.
Soil pH meters can provide a rough measurement of water pH, but are limited to emergencies or scenarios where accuracy is not critical.
After measuring acidic soils (pH<5.5), the electrode needs to be additionally immersed in a weak alkaline solution (pH 9~10) to prevent the “acid memory effect”;
Long-term use in water measurements accelerates electrode deterioration, so it is recommended that a dedicated device be used for this purpose!

How Much Does a pH Meter Cost?
It wouldn’t be right to mention one fixed amount.
The pH meter price will vary from model to model.
To know the exact price, you can ask your preferred manufacturer to provide a quotation for the type of good pH meter you want.

How Often Should You Calibrate a pH Meter?
The calibration frequency is not a fixed value, the recommendations are:
Laboratory: Before each experiment
Industrial: At the beginning of each shift + midway through slope verification
Field: First time of day + in case of abnormal readings
Accurate recording of slope and temperature for each calibration is more important than simply increasing the frequency!

How To Clean The pH Meter/Probe/Electrodes?
After contamination of the bulb and the liquid junction, the electrode is first cleaned with the following solvents, then the solvent is washed off with deionized water, and the electrode is immersed in the soaking solution for activation.
pH pollutant cleaning agent:
Inorganic metal oxides less than 1 mol / L dilute acid; organic oil and grease substances dilute detergent (weak acidic); resin polymer substances dilute alcohol, acetone, ether; protein hemocyte precipitates acid enzyme solution (such as the mother of raw tablets) pH pigmented substances dilute bleach, hydrogen peroxide.

Which Electrode Used In pH Meter?
| Reference electrode | Electrodes that do not respond to the activity of hydrogen ions in solution and have a known and constant electrode potential are called reference electrodes. There are several types of reference electrodes such as pH mercury sulfate electrode, calomel electrode and silver/silver chloride electrode. |
| Indicator electrode | An electrode that responds to the activity of hydrogen ions in a solution and changes the electrode potential is called a pH indicator electrode or pH measurement electrode. There are several types of pH indicator electrodes, such as hydrogen electrode, antimony electrode and glass electrode, but the most commonly used is the glass electrode. Glass electrode is a pH glass rod, as well as by the special composition of the composition of the glass membrane sensitive to hydrogen ions. The glass membrane is generally bubble-shaped, bubble pH filled with internal reference solution, inserted into the reference electrode (generally silver / silver chloride electrode). A pH indicator electrode alone cannot be measured, it must be measured together with the reference pH electrode. |
| Composite electrode | The combination of pH glass electrode and reference electrode is called pH composite electrode. If the shell is plastic, it is called plastic pH shell pH composite electrode. The biggest advantage of composite electrodes is that they are all in one, and pH is easy to use. The structure of the pH composite electrode is mainly composed of electrode bulb, glass support rod, internal reference electrode, internal reference solution, pH shell, external reference electrode, external reference solution, liquid boundary, electrode cap, electrode wire, socket and other components. |

How Long Does A Typical pH Meter Electrode Last?
The normal use of pH meter electrode/probe is half a year to one year, the use of not good condition of industrial electrode is shorter.
The life of the electrode is affected by the following factors
Using medium: the same electrode will contact with different mediums, some mediums are very clean, such as tap water, and some mediums are strong acid and alkali or even high temperature.
Time of use: occasional use and frequent use will lead to different life cycle of the electrode.
Whether to clean and maintain: can significantly extend its life and ensure data reliability.

How Should I Store My Electrode?
1. When the instrument is not in use, rinse the electrode with distilled water to minimize contamination and keep the pH probe in a protective cover with HI70300 storage solution. Do not soak or store the electrode in distilled or deionized water.
2. If the pH electrode is dry, soak it in HI70300 storage solution for at least one hour to reactivate the electrode.
3. To prolong the life of the pH electrode, it is recommended that it be immersed in an electrode cleaning solution for a half-hour soak and clean every month, then rinsed clean again with distilled water and recalibrated for storage.

How To Maintain pH Meter?
Daily maintenance of aquarium pH meter includes:
Make sure the sponge inside the cap of the electrode moist, add a few drops of pure water if dry.
When the electrode head is dry, it can be immersed in maintenance solution for a few hours;
Wash the electrode head after use and keep it clean;
Avoid hitting the electrode head;
Calibrate the electrode head regularly, it is recommended to calibrate the electrode head once a week, and daily calibration is required for everyday use;
Soak the electrode head in the maintenance solution regularly to keep it in an activated state.

How To Store And Use pH Standard Buffer Solution?
Preservation: glass bottle/polyethylene bottle (PE bottle for alkaline buffer solution), sealed and refrigerated (5~10℃), shelf life of about 6 months.
-Discard when turbid, precipitated or moldy.
-Use: Dispense into 50mL vials, equilibrate at room temperature for 1~2 hours and then use to avoid contamination by pouring back into larger vials.
- pH 9.18, 10.01 is easy to absorb CO2, it is recommended to use up within 2~3 days; pH 7.00 and other higher stability.

How To Make Buffer Solution For pH Meter?
When preparing buffer solutions, the deionized water used to make the solution should be boiled for 15-30 minutes to remove dissolved carbon dioxide. Avoid contact with air during cooling to prevent contamination by carbon dioxide.
The buffer solution should be stored in glass or polyethylene bottles. The lid should be tightly closed and the bottle stored in a low-temperature environment of 5-10°C. It can be stored for about six months. If turbidity, mildew, or sedimentation is found, it should not be used. Buffer solutions prepared in an environment higher than 10°C can be used for two to three days. Solutions with a pH of 7.00, 6.86, or 4.00 can be used a little longer. Due to the absorption of carbon dioxide in the air, solutions with a pH of 9.18 or 10.01 are more likely to change pH values, so it is recommended to use them as soon as possible.
When using the buffer solution, pour the solution into a 50 mL polyethylene vial and leave it at ambient temperature for 1-2 hours to equilibrate before use. Do not re-pour the solution from the vial into a larger vial to avoid contamination of the reagents.

What Do You Use To Calibrate a pH Meter
Calibration of electronic pH meter must be done using standard buffer solutions (Buffer Solutions) that are highly pH stable and certified to international standards at specific temperatures. pH 7.00 + (4.01 or 10.01) → covers 90% of application scenarios.

What Is ATC In pH Meter?
ATC: Automatic temperature compensation: Laboratory pH meters are equipped with automatic temperature compensation. This is usually accomplished by a separate temperature probe (or integrated in a composite electrode). The probe measures the actual temperature of the solution, T. The instrument calculates the theoretical slope at that temperature (2.303 RT / F) in real time based on the Nernst equation and adjusts its internal calculations accordingly.
The ATC compensates for the change in the slope of the electrode response with temperature. It ensures that the potential-pH relationship curve established by the instrument during calibration scales correctly to the current temperature.

What Is mV In pH Meter?
mV is the voltage signal directly generated by the pH electrode that reflects the H⁺ activity of the solution. The mV (millivolt) that appears in pH measurement refers to the voltage signal output from the pH electrode, which is the core raw data of pH measurement. pH value, which is the value of the instrument internally based on the millivolt (mV) voltage signal generated by the electrode, after complex temperature compensation, Nernst's equations, and calibration data, will be converted from the measured mV value to the pH value required by the user.

What Is ORP In pH Meter?
ORP, or Oxidation Reduction Potential, is a parameter independent of pH, but is strongly influenced by pH.
It is measured using an ORP electrode (platinum electrode) and the result, expressed in mV, reflects the strength of the oxidizing or reducing properties of the solution.
On a multi-function water analyzer or digital pH test meter with mV mode, the mV reading displayed when the ORP electrode is connected or switched to the ORP/mV function is the ORP value.

What Is The Difference Between a pH Meter And a pH Paper?
| pH Meter | Using electrodes, based on Nernst's equation to measure the potential, the measurement does not need to consume H + or OH, and can more correctly reflect the actual pH value of the solution. |
| pH Paper | Based on the color development reaction of the indicator, the test paper contains a certain amount of indicator, and it needs to consume a certain amount of H + or OH to change from one color to another. If there is not enough H + or OH in the solution to change the color of the indicator, the pH paper may not accurately show the pH of the solution, especially if it is outside the buffer range, and the pH paper value may deviate significantly from the actual pH of the solution. |

What Are The Main Types Of pH Meter?
| Pen pH Meter | Features: compact and lightweight, shaped like a pen, integrated electrode and host, waterproof design. Applicable scenarios: field water quality testing, rapid on-site measurement (such as ponds, swimming pools, food processing site). Advantages: simple operation, easy to carry, battery-powered. Disadvantages: relatively low precision (usually ± 0.1 pH), simple function. |
| Portable/Handheld pH meter | Features: host and electrode separated, host can be handheld, with display and keys. Applicable scenes: environmental monitoring, industrial inspection, agricultural soil testing. Advantages: higher accuracy (±0.01 pH), support temperature compensation, data storage and other functions, suitable for mobile use. |
| In-line pH meter | Industrial process monitoring) Features: electrode fixed installation in the pipeline or reaction tank, real-time continuous monitoring. Applicable scenes: chemical production, sewage treatment, boiler water monitoring, food and beverage production line. Advantages: 24 hours real-time data output, high pressure/corrosion resistant, supports remote transmission and alarm function. |
| Benchtop pH meter | Laboratory grade Features: host placed on the laboratory bench, need to connect external electrodes, feature-rich. Applicable scenes: laboratory precision analysis, scientific research, quality control (such as pHarmaceutical, chemical, food testing). Advantages: high accuracy (±0.001 pH), multi-parameter expansion (can be connected to ORP, conductivity electrodes), automatic calibration, data export. |

What Factors Affect pH Measurement Accuracy?
- Electrode Aging: After a period of time, the glass membrane of a pH electrode will gradually age, resulting in a slower response time of the electrode and a decrease in the accuracy and stability of the measurement. For example, an electrode used for many years may take longer time to reach a stable potential value, making the measurement results biased.
- Electrode contamination: If the electrode surface is contaminated by oil, protein, particles, etc., it will affect the exchange process of hydrogen ions, thus affecting the measurement results.
- Inaccurate instrument calibration: pH machine water needs to be calibrated regularly with standard buffer solution, if the calibration is inaccurate, the measurement results will produce errors
- Temperature: Temperature has a significant effect on pH measurement. On the one hand, temperature affects the slope of the electrode, and on the other hand, temperature changes the equilibrium constant of the chemical reaction in solution, thus affecting the hydrogen ion concentration
- Ionic Strength: The ionic strength of a solution affects the activity coefficient and therefore the pH measurement. When the ionic strength of the sample solution differs significantly from the ionic strength of the calibration solution, the measurement results will be in error. For example, when measuring the pH of a high-salt solution, the measured value may deviate from the actual value due to the ionic strength.
- Homogeneity of the sample: If the sample is not homogeneous, such as the presence of precipitation, stratification and other phenomena, it will lead to inaccurate measurement results. When measuring, it is necessary to ensure that the sample is well mixed. For example, when measuring soil leachate containing suspended particles, if it is not shaken well, the measurement results may vary depending on the sampling location.
- Measurement time: Measurement needs to wait for the electrode to reach a stable potential value, if the measurement time is too short and the electrode has not reached equilibrium, the measurement result will be inaccurate. If the measurement time is too short, the electrode has not reached equilibrium, the measurement results will be inaccurate. Different solutions and electrodes reach equilibrium in different times, generally need to wait for several minutes
- Stirring speed: It is necessary to control the appropriate stirring speed during the measurement.
- Depth of electrode insertion: The depth of electrode insertion into the solution will also affect the measurement results. The electrode should be inserted into the solution at a depth where the glass membrane is completely submerged.

What Is The Precautions When Using pH Meter?
1. The electrode socket should be kept dry.
2. The input should be short-circuited when measurement is not carried out.
3. New electrodes or electrodes that have not been used for a long time must be soaked in distilling water for a few hours before using.
4. The electrode bulb should be fully immersed in the solution to be measured when measurement is carried out.
5. When used, the internal reference electrode should be immersed in the internal reference solution.
6.When using, the rubber plug of the electrolyte filling port of the reference electrode should be pulled out.
7. Potassium chloride salt bridge solution should be added frequently to keep the liquid level higher than the silver/silver chloride wire.

How To Buy An pH Meter From China?
Importing high performance electronic pH tester from China can be difficult for those who are new to international imports.
Purchasing from a reputable manufacturer and supplier eliminates hassle of logistics, as they handle the safe and reliable delivery of the product directly to your place.
When buying the pH meter instrument, it is important to keep the following points in mind:
1. Confirm that your selected supplier can securely export and deliver the machine, and ensure that your agreement includes all essential shipping information.
2. Choose the delivery way (by sea, air, or express) based on your preference.
3. Remember to factor in duty imports and taxes when calculating the total cost.

How Does a Manufacturer Deliver The pH Meter?
Safe shipping is so important as quality manufacturing.
A responsible manufacturer should deliver your rapitest pH meter safely.
Most water pH meter manufacturers use export cartoon case to pack the device.
You should read all the necessary instructions available on the sides of the boxes before boxing.

Is There Any Warranty On pH Meter?
For optimal performance, it is important to regularly maintain your food pH meter, so it can operate efficiently for an extended period. It is essential to be aware of the warranty coverage for your equipment, as it ensures that any manufacturing issues will be repaired at no additional cost by the supplier. Therefore, it is recommended to purchase a pH meter near me from a manufacturer that offers warranty services —Cialan.
Reputable digital water pH tester manufacturers typically provide a 1-year warranty. for example, offers complimentary repair and maintenance services during the first year.
Before purchasing a pH water meter, it is advisable to review the warranty period and other associated terms and conditions.

What After-Sale Service Does A pH Meter?
The reliable China digital pH test meter suppliers offers the following after-sale services:
1. Secure product delivery.
2. Thorough inspection prior to installation.
3. Complimentary online guidance for device installation and maintenance.
4. Free training for your users.
5. Regular assessments of your electronic pH meter.

Can You Do OEM&ODM For pH Meter?
Yes, Cialan can do OEM#OEM for our users, just send us your requirements for pool ph meter.

Where To Find pH Meter Manufacturers?
Numerous online directories can connect you with electronic pH tester manufacturers.
A simple search for "top-rated pH test meter manufacturers" yields multiple options. However, it's essential to exercise caution when selecting a manufacturer from online search results. Before making a decision, conduct thorough research on the company's background and reputation. Look up reviews on “Google” and reputable review sites to gauge their credibility.
Additionally, It's also crucial to review their portfolio of request that potential manufacturers share examples of their previous work to ensure they meet your standards.

How To Choose A Right pH Meter?
1. Testing accuracy
This is an important parameter of a water pH machine. Small number, high accuracy.
2. Testing range
The pH measurement range varies greatly for different samples and application scenarios. When purchasing, please make sure that the measurement range of the pH meter can cover the possible pH range of the sample to be measured, so as to avoid the situation of not being able to measure accurately due to out-of-range.
3. Electrode performance
Common electrodes include glass electrodes and composite electrodes. Glass electrodes react strongly to pH but are fragile; composite electrodes are the most common, integrating the measurement electrode and reference electrode together, easy to use and suitable for most aqueous solutions.
4. Calibration function
The instrument can automatically recognize the standard buffer and carry out calibration, which is very convenient and reduces human error. It can also be calibrated at single and multiple points to meet different measurement needs.
5. Ease of use
Simple operation interface, easy to read, portable and easy to maintain.

What Should You Look For In The Best pH Meter?
The best pH meter is one that is stable, reliable, easy to operate, rugged (for your environment), at the level of accuracy you need, and has reasonable maintenance costs.